Blood Pressure Drugs Recalled Over Cancer Concerns
COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer. In 2018 and 2019 it has seemed like news about potential cancer-causing.
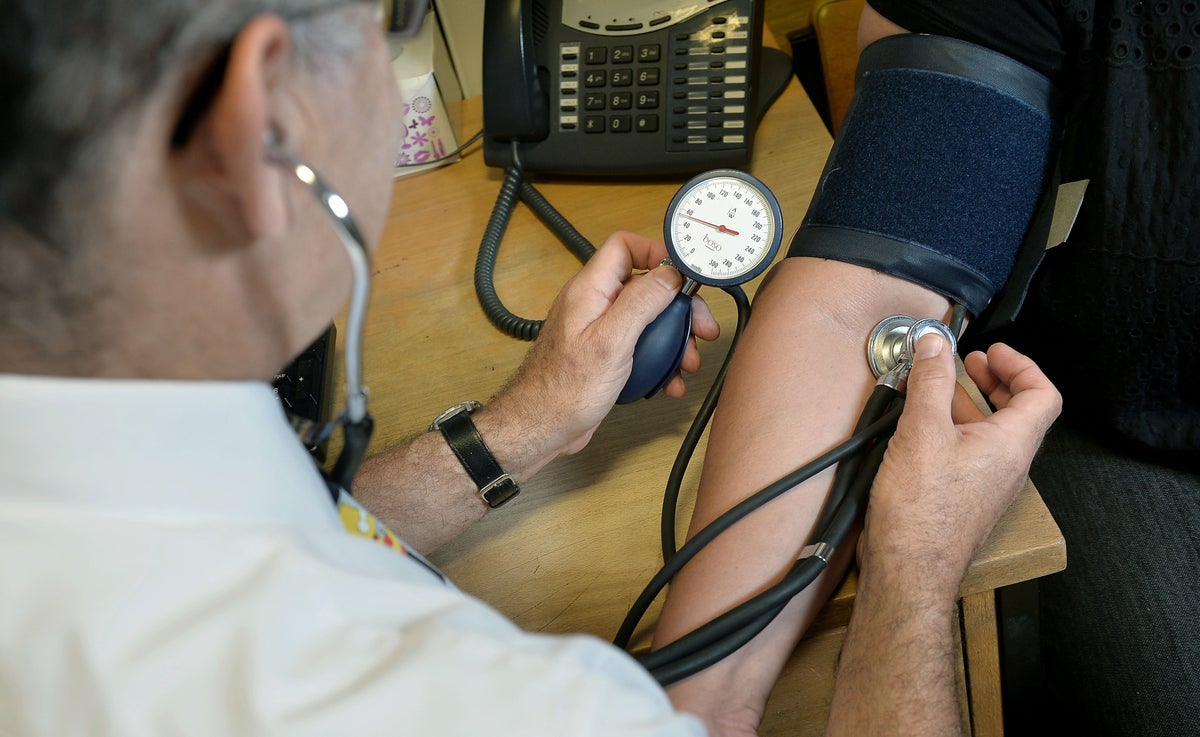
Blood Pressure Pills Recalled Over Cancer Risk The Independent
The FDA has published a new recall advisory from Lupin Pharmaceuticals over two different drugs.
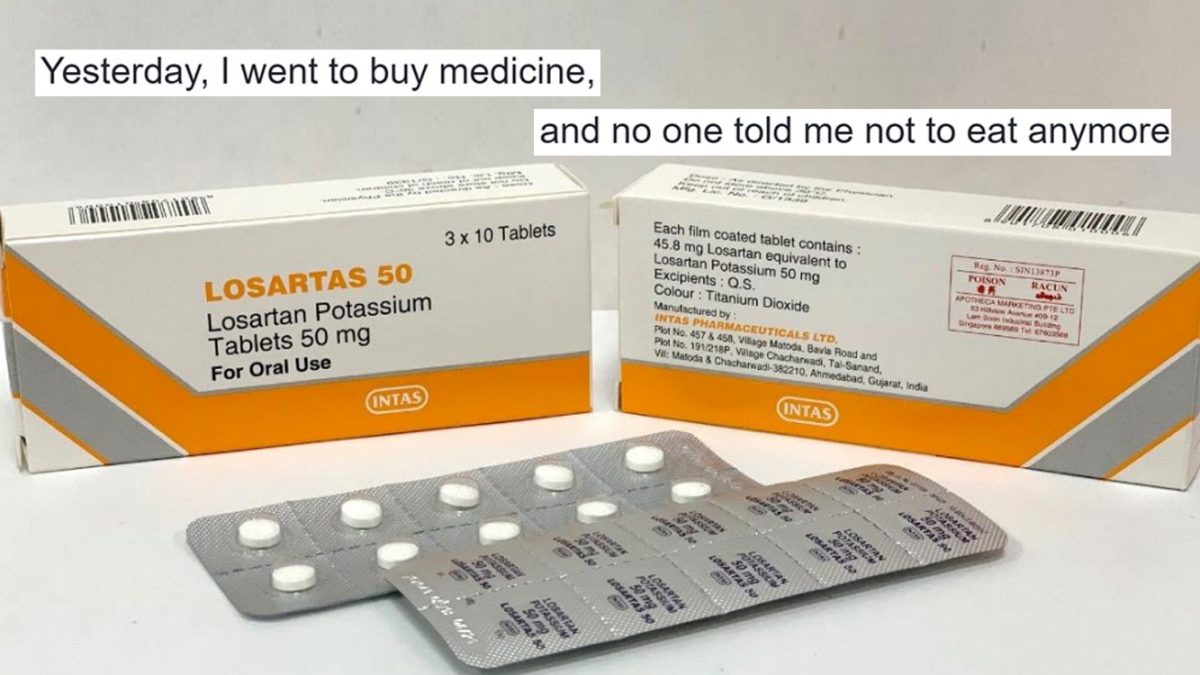
Blood pressure drugs recalled over cancer concerns. Roston - Oct 15 2021 744pm CDT. Blood pressure medication recalled over risk of cancer-causing impurity Cleveland Ohio 2 hours ago Man convicted of killing wife. This time Torrent Pharmaceutical Limited is recalling additional lots of Losartan Potassium tablets which is used to treat hypertension as well as hypertensive patients with Left.
Blood pressure and fluid retention drugs recalled over cancer concerns. 9 hours agoLupin Pharmaceuticals Inc. The additional lots add to the lots of blood pressure medications that have been recalled in the past 14 months or so.
See the List of Blood Pressure Heart Drugs Recalled Over Cancer Concerns The FDA is urging patients to look at the name of the drug and company on the label of a prescription bottle to determine. Blood pressure and fluid retention drugs recalled over cancer concerns. Torrent Pharmaceuticals Limited has again expanded a recall of its blood pressure medication over concerns the tablets contain small amounts of a cancer-causing ingredient.
Blood pressure pills recalled over cancer risk. Is voluntarily recalling a blood pressure medication that possibly contains high levels of a cancer-causing impurity according to the US. More than 30 batches of pills used to treat high blood pressure have been contaminated by an impurity that can increase the risk of cancer.
The company which stopped marketing these particular drugs in January says it is recalling the batches due to potentially higher than acceptable levels of an impurity called N-nitrosoirbesartan. Qantas charged in. The recall covers the.
Blood pressure and fluid retention drugs recalled over cancer concerns October 16 2021 Renata Simons The FDA has published a new recall advisory from Lupin Pharmaceuticals over two different drugs. Patients are being advised not to stop taking their medication as it. A generic drug company has recalled three commonly prescribed blood pressure medications over concerns they could include small amounts of a cancer-causing impurity.
The Food and Drug Administration has expanded a recall on high blood pressure medications due to potential cancer risk. After the companys testing process it detected high levels of a cancer-causing substance in the affected products. In another recall over cancer-causing impurity levels Lupin Pharmaceutical has recalled several batches of its Irbesartan tablets and Irbesartan and Hydrochlorothiazide tablets.
Macleods Pharmaceuticals Limited is recalling a lot of Losartan PotassiumHydrochlorothiazide combination tablets because they may contain a cancer-causing s. Another blood pressure medication has been recalled over concerns it could contain trace amounts of carcinogens. Pharmaceutical company recalls blood pressure medications over possible cancer-causing impurity.
Since July the US. The FDA has published a new recall advisory from Lupin Pharmaceuticals over. The FDA has published a new recall advisory from Lupin Pharmaceuticals over two different drugs.
The recalled drugs all include. Two more blood pressure medications recalled over cancer concerns. Food and Drug Administration has announced voluntary recalls from Major Pharmaceuticals Solco.
Fears that some drugs have been contaminated with a substance that could possibly increase the risk of cancer has seen certain blood pressure drugs recalled. Pharmaceutical firm Mylan is dramatically expanding a nationwide recall of some blood pressure medications after detecting trace amounts. Blood Pressure Medication Recalled Over Cancer Concerns.
On the other hand in this recent recall Lupin Pharmaceuticals recalled two types of its blood pressure medications. As a result users of the affected devices may suffer from lung injuries respiratory problems and even cancer. The recall has been issued by the Medicines and Healthcare products Regulatory Agency MHRA and concerns 25 batches of Irbesartan-containing medications.
Blood pressure drugs recalled over fears they contain cancer-causing chemical. Mylan recalls blood pressure drugs over cancer concern. 26 likes 70 shares.
Latino Catholics have one of the highest COVID-19 vaccination rates in the US. The UK medicine regulator today issued a recall for 31 batches of.
Lawsuits Flow In Over Tainted Blood Pressure Drugs Biospace

Blood Pressure Medications Needed During Covid 19
Drugs Recalled With Possible Carcinogen Contamination Ctca

Batches Of High Blood Pressure Drug Recalled Due To Contamination Nationalworld
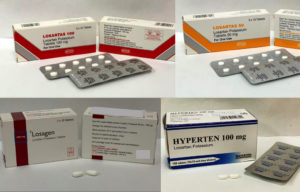
3 Brands Of High Blood Pressure Medication Losartan Recalled For High Levels Of Cancer Causing Contaminant Dr Siew Com

Recall Of Blood Pressure Medication Losartan Expands Over Cancer Concerns Everyday Health
.jpg)
Valsartan Losartan Irbesartan Causing Cancer

Another Blood Pressure Med Recall For Cancer Concerns What You Should Do

Losartan And Irbesartan Tablets Recalled Amid Cancer Fear News The Sunday Times

Fda Recall Of Heart Meds Grows Due To Cancer Risk Everyday Health
Teva Recalls More Tainted Blood Pressure Drugs Biospace
Blood Pressure Pills Used By Millions Of Brits Recalled Over Cancer Risk Fears Mirror Online

Amlodipine And Valsartan Recalls Lawsuit Implications

Blood Pressure Meds Recalled Over Contamination Concern Wfmj Com News Weather Sports For Youngstown Warren Ohio

S Porean Who Took High Blood Pressure Drug For 2 Years Wants Answers After Hsa Recall

Latest Blood Pressure Medication Recall List Updated September 2019 Oregonlive Com
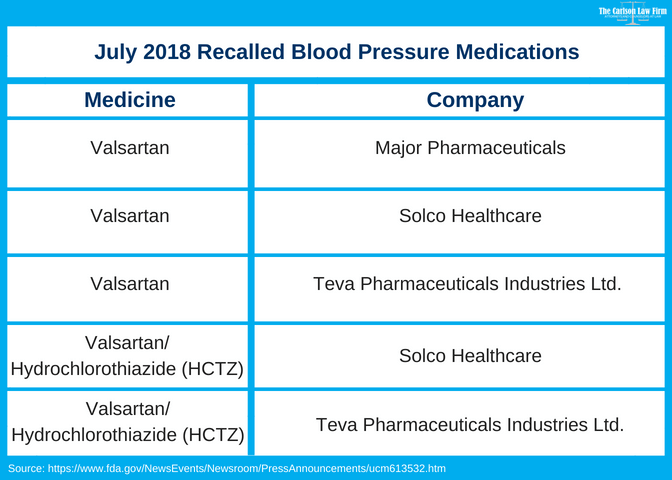
Valsartan Recall Fda Finds Carcinogenic Impurity In Blood Pressure Meds

